Modification of Ni–P alloy coatings for better hydrogen production by electrochemical dissolution and TiO2 nanoparticles†
Abstract
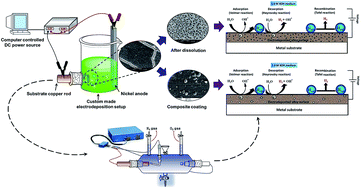
This work reports the modification of Ni–P alloy coatings for better hydrogen production by electrochemical dissolution and TiO2 nanoparticle incorporation. The first part is devoted to optimization of a new citrate bath for the development of an efficient electroactive Ni–P electrode material by electrodeposition, using glycerol as an additive. The Ni–P alloys developed at 4.0 A dm−2 and 2.0 A dm−2 were found to be good for the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER), respectively as demonstrated by cyclic voltammetry (CV) and chronopotentiometry (CP) methods. The Ni–P alloy showing good catalytic activity for HER is found to be less active for OER and vice versa. The unique electrocatalytic property of the coatings was attributed to its structure, morphology and composition, confirmed by XRD, SEM and EDS analyses. In the second part, the electrocatalytic activity of Ni–P alloy coatings for HER has been improved further by anodic dissolution and TiO2 nanoparticle incorporation. Drastic improvement in the electrocatalytic activity for HER was found in both anodically treated and Ni–P–TiO2 composite coatings, compared to as-coated Ni–P alloys. The highest electrocatalytic character of the Ni–P–TiO2 composite coating was attributed to a greater number of electroactive centres, affected by TiO2 nanoparticle incorporation, and experimental results are discussed.

 Please wait while we load your content...
Please wait while we load your content...