Synthesis and characterization of TPGS–gemcitabine prodrug micelles for pancreatic cancer therapy†
Abstract
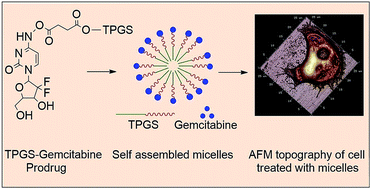
The therapeutic potential of a nucleoside analog, gemcitabine, is severely compromised due to its rapid clearance from systemic circulation by enzymatic degradation into an inactive metabolite. In the present investigation, micelles based on polymer–drug conjugate were developed for gemcitabine and investigated for their potential to improve cancer chemotherapy. The tocopherol poly(ethylene glycol) succinate 1000 (TPGS)–gemcitabine prodrug was synthesized via an amide linkage and characterised by analytical methods, including FT-IR, 1H NMR, and MALDI-TOF. The micellar formulation of TPGS–gemcitabine prodrug was developed by a self-assembly technique and evaluated for various physicochemical parameters including particle size, polydispersity, morphology, critical micelle concentration and release profile. It was observed that gemcitabine present in TPGS–gemcitabine micelles was resistant to deamination by crude cytidine deaminase. The improved cytotoxicity of the micellar formulation was observed using TPGS–gemcitabine micelles against pancreatic cancer cells. Further, it was found that, unlike native gemcitabine, nucleoside transporters were not required for TPGS–Gem micelles to demonstrate their anticancer potential. These findings revealed that TPGS–gemcitabine micelles may serve as a promising platform for gemcitabine in order to improve its anticancer efficacy.

 Please wait while we load your content...
Please wait while we load your content...