Competitive adsorption of VOCs from binary aqueous mixtures on zeolite ZSM-5†
Abstract
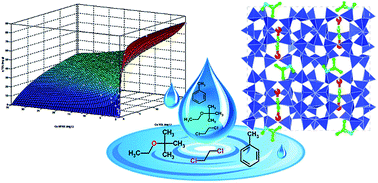
Adsorption equilibria of methyl tert-butyl ether (MTBE)/toluene (TOL), and 1,2-dichloroethane (DCE)/MTBE binary mixtures in aqueous solution on ZSM-5 were measured over a wide range of concentrations. In comparison with the single-component data, the loading of all of the three compounds was reduced in the presence of a second component in the mixture. The binary system was described by a competitive dual site Langmuir adsorption isotherm. The model was chosen on the basis of the results obtained from X-ray diffraction studies of the adsorbent material loaded with equimolar binary mixtures. Rietveld structure refinements provide information about the relative position of molecules inside the structure after TOL-MTBE and DCE-MTBE mixture adsorption. The short intermolecular distances between the adsorption sites of MTBE, DCE and TOL inside the zeolite framework clearly prevent the simultaneous occupancy of one site by more than one component, when these compounds are adsorbed from binary mixtures.


 Please wait while we load your content...
Please wait while we load your content...