Structure guided design and binding analysis of EGFR inhibiting analogues of erlotinib and AEE788 using ensemble docking, molecular dynamics and MM-GBSA†
Abstract
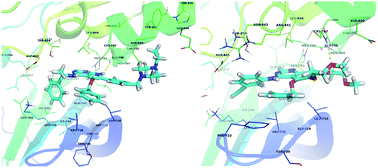
Epidermal growth factor receptor (EGFR) is an important validated drug target for cancer therapy. Some therapeutic agents targeting EGFR have been developed, however these are sometimes ineffective, which calls for the discovery of alternative novel anti-EGFR inhibitors. Thus, we performed ensemble molecular docking and molecular dynamics (MD) against multiple conformations of EGFR to identify, design and validate new analogs of erlotinib and AEE788 that have enhanced binding affinity. To achieve this, curcumin analogues with diverse groups were studied against EGFR using ensemble molecular docking and MD. Based on the preferred orientations and MM-GBSA calculations of the selected top-scoring consensus lead inhibitors, we derived the pharmacophoric features that possess maximum binding affinity for the EGFR binding site. Using these pharmacophoric properties we designed new analogues of two known co-crystallized therapeutic inhibitors of EGFR (erlotinib and AEE788). We then tested the hypothesis using MD that the identified pharmacophoric property increases binding affinity and increases the stability of the designed analogues. Our MD simulations and MM-GBSA calculations confirmed that the occupancy of sub-pocket 2 in the EGFR active site is important for tight EGFR–inhibitor binding. The study thus provides an essential pharmacophoric requirement for design and discovery of new lead candidates as EGFR inhibitors.

 Please wait while we load your content...
Please wait while we load your content...