Kinetic analysis of Fe3+ reduction coupled with nitrate removal by Klebsiella sp. FC61 under different conditions
Abstract
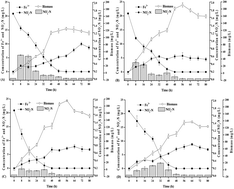
Klebsiella sp. FC61, a newly found iron-reducing bacterium, has the ability of simultaneously reducing Fe3+ and nitrate under different pH and temperature conditions. Based on kinetic equations, the maximum rate of Fe3+ reduction was 0.26 mg L−1 h−1 at pH 6.0 and 0.24 mg L−1 h−1 at 30 °C. The maximum rate of nitrate removal was 0.21 mg L−1 h−1 at pH 7.0 and 0.82 mg L−1 h−1 at 35 °C. After 56 h, nitrate was removed completely, coupled with Fe3+ reduction, and there was a transient accumulation of nitrite. Fe3+ and nitrate reduction followed the selected kinetic equations well. Moreover, the Arrhenius equation further confirmed the reliability of the data under the different temperature conditions. When analyzed using XRD and SEM, a form of crystalline iron oxide with recovered deposits of strain FC61 was observed.

 Please wait while we load your content...
Please wait while we load your content...