Accessing Centnerszwer's quasiracemate – molecular shape controlled molecular recognition†
Abstract
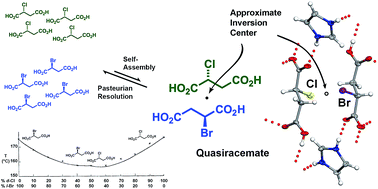
M. Centnerszwer's seminal 1899 report investigated the stereochemical relationship between optical antipodes of different substances using melting-point behavior. One intriguing melting-point phase diagram produced from this early investigation combined (+)-2-chlorosuccinic acid [(+)-1] and (−)-2-bromosuccinic acid [(−)-2]. While Centnerszwer's data clearly indicates the formation of a quasiracemic phase – i.e., materials constructed from pairs of isosteric molecules of opposite handedness – at the 1 : 1 component ratio, this material is energetically less favorable than the chiral counterparts. The consequence of this crystal instability is significant as evident by the absence of literature sited crystal structures for the quasiracemic phase (+)-1/(−)-2 and racemates (±)-1 and (±)-2. This study circumvented this challenge by generating multi-molecular assemblies using additional crystallizing agents capable of complementing the hydrogen-bond abilities of succinic acids 1 and 2. Both imidazole (Im) and 4,4′-bipyridyl-N,N′-dioxide (BPDO) served as tailor-made additives that effectively modified the crystal packing landscape of quasiracemate of (+)-1/(−)-2. Combining imidazole with the quasiracemate, racemate, and enantiopure forms of 1 and 2 resulted in crystal structures characterized as molecular salts with layered motifs formed from highly directional N+–H⋯carboxylate and carboxyl⋯carboxylate interactions. In contrast to the enantiopure [(+)-1·Im and (−)-2·Im] and racemic [(±)-1·Im and (±)-2·Im] systems, neighboring molecular layers observed in quasiracemate (+)-1/(−)-2·Im are organized by approximate inversion symmetry. Assessment of the crystal packing efficiency for this series of molecular salts via crystal densities and packing coefficients (Ck) indicates imidazole greatly alters the crystal landscape of the system in favor of racemic and quasiracemic crystal packing. A similar desymmetrized crystal environment was also realized for the ternary cocrystalline system of (+)-1/(−)-2·BPDO where the components organize via N+–O−⋯carboxyl contacts. This study underscores the importance of molecular shape to molecular recognition processes and the stabilizing effect of tailor-made additives for creating new crystalline phases of previously inaccessible crystalline materials.

 Please wait while we load your content...
Please wait while we load your content...