α-Chymotrypsin superactivity in quaternary ammonium salt solution: kinetic and computational studies
Abstract
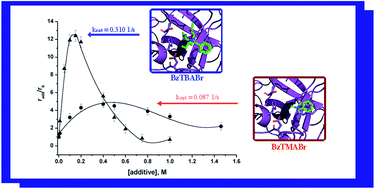
As previously reported, quaternary ammonium salts with bulky hydrophobic portions provoke a superactivation of α-chymotrypsin in aqueous solution: this is the case of the surfactant cetyltributylammonium bromide (CTBABr) and the corresponding salt tetrabutylammonium bromide (TBABr). In order to achieve a broader knowledge of the enzyme–additive interactions, in this paper the activity and stability of α-chymotrypsin were tested in the presence of additives with slightly modified bulky ammonium groups. The effect of three additives with a benzylic group as substituent (benzyltrimethylammonium bromide (BzTMABr), benzyltributylammonium bromide (BzTBABr) and benzyldodecyldimethylammonium bromide (BzDDABr)) was investigated. A significant increase in instantaneous activity, but a deactivation of enzyme, faster than in pure buffer, was observed. Moreover, two novel dicationic salts, (1,8-bis(tributylammonium)octane dibromide (bisBOAB) and 1,4-bis(tributylammonium)xylene dibromide (bisBAB)) were designed and synthesized in order to evaluate the effect of two tributylammonium head groups with a different spacer. BisBOAB provoked superactivation and stabilization effects in a way similar to the “homologue” TBABr, but at lower concentration. In contrast, when the benzyl group was constrained within the spacer structure, the obtained superactivity was lower than in the presence of a more flexible hydrocarbon chain spacer, and enzyme deactivation was faster. Molecular modelling studies allowed us to rationalize the hypotheses derived from kinetic evidence. The results confirmed that the improvement in the catalytic properties observed in the presence of additives with a bulky, hydrophobic ammonium head group could be addressed to an increase in the overall hydrophobicity of the α-chymotrypsin catalytic site.


 Please wait while we load your content...
Please wait while we load your content...