Self-assembled structural transition in l-Arg/H-AOT mixtures driven by double hydrogen bonding†
Abstract
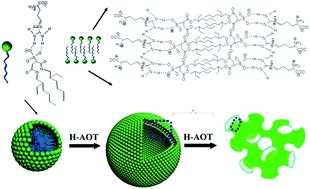
The aggregation properties of L-arginine (L-Arg) with the acidic form of sodium bis(2-ethyl-1-hexyl)sulfosuccinate, H-AOT, in water, were investigated in detail. Double hydrogen bonding formed between the guanidyl group on the L-Arg molecule and the –SO3H group on the H-AOT molecule. The combination of L-Arg and H-AOT through hydrogen bonding and electrostatic interaction led to the self-assembly of the mixtures. The addition of hydrophobic H-AOT to L-Arg solution modulated the hydrophobicity/hydrophilicity of the L-Arg/H-AOT complexes, resulting in an increase of the packing parameter. Accordingly, a transition of the self-assembled structure from micelles to a closed lamellar bilayer structure (vesicles) and then a bicontinuous bilayer structure (sponge phase) occurred. The microstructural transition was reflected by rheological measurements, for which significant changes in both viscosity and elasticity appeared, and this was further confirmed by Cryo-TEM and FF-TEM observations.

 Please wait while we load your content...
Please wait while we load your content...