Chromogenic and fluorescence sensing of pH with a Schiff-base molecule†
Abstract
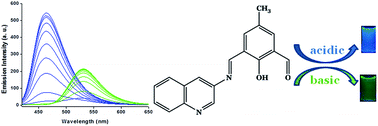
pH is one of the key parameters of many chemical and biological processes and also to assess the quality of water. We have synthesized and characterized a new Schiff-base molecule, 2-hydroxy-5-methyl-3-((quinolin-3-yliminio)methyl)benzaldehyde (HMQB) which is able to detect different pH regions. It shows a strong emission band at 464 nm in Britton Robinson buffer solution of pH 2.0 upon excitation at 400 nm. With the increase in pH value of the medium, the emission intensity at 464 nm decreases gradually and at the same time, a new fluorescence peak emerges at 530 nm. The UV-vis spectra of HMQB exhibits peaks at 350 nm and 435 nm in acidic and basic regions respectively. Images of HMQB in different pH solutions indicate no coloration and yellowish green coloration in acidic and basic media respectively corroborating the results of the UV-vis spectra. We have applied the probe molecule successfully to sense pH regions of river water and lemon juice. HMQB has also been used for cell imaging study.

 Please wait while we load your content...
Please wait while we load your content...