Racemic alkaloids from Macleaya cordata: structural elucidation, chiral resolution, and cytotoxic, antibacterial activities†
Abstract
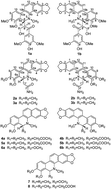
Three pairs of new enantiomeric natural alkaloids (±)-macleayins C–E (1–3), together with five pairs of known racemic alkaloids (4–8), were isolated from the aerial parts of Macleaya cordata. Compounds 1–5 were separated successfully by chiral-phase HPLC to yield optically pure isomers. However, chiral resolution of compounds 6–8 existing as racemers in the plant was unsuccessful. It is noteworthy that, macleayin C represents a novel type of hybrid composed of a dihydrobenzophenanthridine alkaloid and a phenylpropanoid. The structures including the absolute configurations of (±)-1–5 were established by detailed spectroscopic analyses and electronic circular dichroism calculations. All the isolates were evaluated for in vitro anti-tumor and antibacterial activities, and compounds (±)-4–5 exhibited potent cytotoxicity against HL-60 cell lines with IC50 values less than 3.0 μM, and compounds (±)-1–3 showed moderate cytotoxic activity. Only compound 7 revealed inhibitory activities against Staphylococcus aureus, Bacillus subtilis, and Candida albicans with MIC values of 33.07, 8.27, and 8.27 μg mL−1, respectively.

 Please wait while we load your content...
Please wait while we load your content...