Effects of nonionic surfactant on the parasitic corrosion of lithium anode in lithium–water battery
Abstract
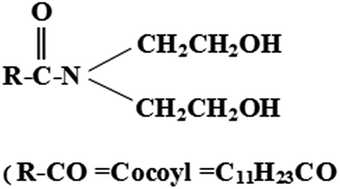
The use of a nonionic surfactant, namely cocamide diethanol amine (CDA), as a parasitic inhibitor for lithium corrosion in 4.0 M LiOH aqueous solution has been evaluated in the 298–313 K temperature range. The efficiency of the CDA surfactant is evaluated by hydrogen evolution at OCP and potentiodynamic polarization measurements. Results show that the CDA surfactant is an appropriate inhibitor for parasitic corrosion. The CDA surfactant slows down the hydrogen evolution reaction during the immersion of lithium in 4.0 M LiOH aqueous solution. The efficiency of the CDA surfactant is promoted with growing surfactant concentration, reaching an extreme value (78.6%) at the critical micelle concentration (CMC). A Tafel polarization study reveals that the CDA surfactant acts as a cathodic-type inhibitor. Adsorption of the CDA surfactant on the lithium surface is unprompted and fits with Langmuir's isotherm. The associated activation energy and thermodynamic parameters are calculated to elaborate the mechanism of corrosion inhibition.

 Please wait while we load your content...
Please wait while we load your content...