Evaluating corrosion inhibition property of some Schiff bases for mild steel in 1 M HCl: competitive effect of the heteroatom and stereochemical conformation of the molecule†
Abstract
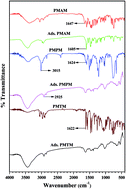
To evaluate the effect produced by the heteroatom present in organic Schiff bases along with overall stereochemical conformation of such bases towards corrosion inhibition performance, three Schiff bases, namely 2-pyridyl-N-(2′-methylamino phenyl) methyleneimine (PMAM), 2-pyridyl-N-(2′-methylthiophenyl) methyleneimine (PMTM) and 2-pyridyl-N-(2′-methoxyphenyl) methyleneimine (PMPM), are assessed for mild steel in 1 M HCl. Chemical similarity exists among these three molecules, varying only in the nature of the heteroatom present away from the imine group. Results from weight loss, potentiodynamic polarization and electrochemical impedance spectroscopic (EIS) techniques demonstrate that the Schiff bases form a resistive layer on the metal surface, and thereby block the cathodic as well as the anodic reaction sites effectively. Experimentally obtained corrosion inhibition efficiency follows the sequence: PMAM > PMTM > PMPM, and this is also confirmed following surface electron microscopy (SEM) images. The Langmuir isotherm is found to provide a sufficiently good description of adsorption behavior of the compounds on the metal surface. Density functional theory (DFT) calculations and molecular dynamics (MD) simulations are done to correlate the efficiency of these compounds with their intrinsic molecular parameters, as well as energy optimized molecular orientation.

 Please wait while we load your content...
Please wait while we load your content...