A comprehensive density functional theory study of the key role of fluorination and dual hydrogen bonding in the activation of the epoxide/CO2 coupling by fluorinated alcohols†
Abstract
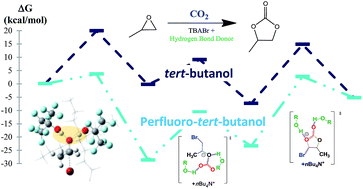
The activation mechanism of the CO2/propylene oxide coupling catalysed by a bicomponent organocatalyst combining the use of TBABr with (multi)phenolic or fluorinated hydrogen bond donors (HBDs) was investigated using Density Functional Theory (DFT). Thus, it was shown that increasing the number of electron withdrawing trifluoromethyl substituents in HBDs strengthens their proton donor capability and allows a better stabilization by hydrogen bonding of the intermediates and transition states. In addition, the high efficiency of fluorinated monoalcohol activators is related to a dual hydrogen bonding mechanism by two fluorinated molecules that cooperatively contribute to the CO2/propylene oxide coupling.

 Please wait while we load your content...
Please wait while we load your content...