Novel reaction of 3,4-dibromofuran with azo diesters to give tetrahydropyridazinones†
Abstract
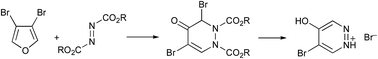
Cycloaddition of 3,4-dibromofuran with azo diesters proceeds by a Diels–Alder reaction followed by a novel rearrangement to give 3,5-dibromotetrahydropyridazin-4-ones. Variable-temperature NMR spectroscopy shows four separate conformations at low temperature attributed to restricted rotation about the carbamate functions. The ethyl compound decomposes upon storage with loss of the carbamate groups and aromatisation to give a simple bromohydroxypyridazinium salt.

 Please wait while we load your content...
Please wait while we load your content...