Facile one-pot preparation of chiral monoliths with a well-defined framework based on the thiol–ene click reaction for capillary liquid chromatography
Abstract
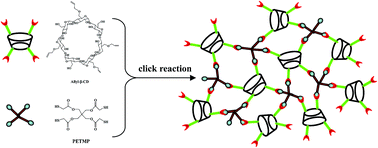
A novel chiral cyclodextrin (CD) monolith was easily prepared via a one-pot process based on the thiol–ene click reaction of allyl-β-CD with pentaerythritol tetra-(3-mercaptopropionate) in a fused-silica capillary. The effects of both the composition of prepolymerization solution and reaction temperature on the morphology, permeability, and selectivity of the β-CD chiral monolith were investigated in detail. The conditions were optimized to fabricate a homogeneous and permeable chiral monolith. In this study, the β-CD monolith was used as the stationary phase of capillary liquid chromatography for the chiral separation of several pharmaceutical enantiomers including flavanone, flurbiprofen, naproxen, synephrine, isoprenaline sulfate, ketoprofen, and atropine sulfate monohydrate. Compared to the previously reported two-step method, this one-pot method for the preparation of a β-CD chiral monolith is simple and time-saving. Moreover, good resolutions were obtained for chiral isomers in a shorter analysis time compared to that reported in the literatures. These results indicate that the thiol–ene click chemistry provides a simple and robust method for the preparation of a chiral β-CD monolith.

 Please wait while we load your content...
Please wait while we load your content...