Structural analogues of Hoveyda–Grubbs catalysts bearing the 1-benzofuran moiety or isopropoxy-1-benzofuran derivatives as olefin metathesis catalysts†
Abstract
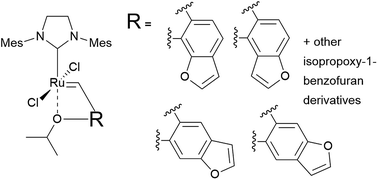
We have used the DFT/M06-D3 computational method to study structures and activation free energies for a series of Hoveyda–Grubbs-like catalysts with the isopropoxybenzene part replaced by 1-benzofuran and ten derivatives of isopropoxy-1-benzofuran. We show that while Ru coordination by the benzofuran oxygen atom is relatively strong, simple modification of isopropoxybenzene by benzofuran doesn't lead to a stable ruthenium complex due to geometrical reasons. Some of the more complex modifications, which replace the phenyl group of the isopropoxybenzylidene with the benzofuran core but leave the isopropoxy group intact, are good candidates for potent metathesis catalysts with free energies of activation of the dissociative path 2–5 kcal mol−1 lower than the Hoveyda–Grubbs catalyst.

 Please wait while we load your content...
Please wait while we load your content...