Facile synthesis of ultrasmall stannic oxide nanoparticles as anode materials with superior cyclability and rate capability for lithium-ion batteries†
Abstract
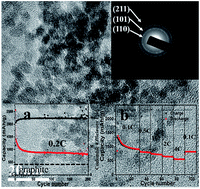
Ultrasmall monodisperse stannic oxide (SnO2) nanocrystals (diameter around 4 nm) with high-performance lithium storage are successfully synthesized via a simple calcination process, during which SiO2 mesoporous nanotubes (SiO2-MNT) are used to play an important role in restraining the growth and aggregation of the nanocrystals. As a kind of anode material for lithium-ion batteries, the as-prepared SnO2 nanocrystals show an excellent electrical performance with a super high reversible capacity of 816 mA h g−1 over 200 charge/discharge cycles at a current density of 160 mA g−1. Moreover, when the current density rises to 3200 mA g−1, the capacity is still as high as 550 mA h g−1, and it can be recovered to 816 mA h g−1 simultaneously when the current density turns back to 80 mA g−1. These results suggest that the obtained SnO2 nanocrystals can achieve a completely reversible transformation from Li4.4Sn to SnO during discharging (i.e., Li is extracted by dealloying and a reversible conversion reaction, generating 6.4 electrons). The superior electrochemical performance can be ascribed to the ultrafine particle size of SnO2, which promotes the reactive activity and alleviates the volume change of the anode composite during charge/discharge cycling.

 Please wait while we load your content...
Please wait while we load your content...