Assembly of substituted phenanthridines via a cascade palladium-catalyzed coupling reaction, deprotection and intramolecular cyclization†
Abstract
The discovery and development of a general method for the one-pot synthesis of substituted phenanthridines is presented. In the presence of Pd(PPh3)4, accessible precursors undergo a Suzuki cross-coupling reaction with 2-(Boc-amino)benzeneboronic acid pinacol ester and then spontaneously undergo deprotection and intramolecular condensation to form the corresponding phenanthridines in one step. This reaction has a wide range of substrates with various functional groups, and the corresponding products have been obtained in good yields.
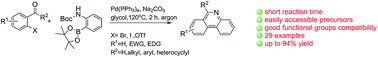

 Please wait while we load your content...
Please wait while we load your content...