Electrochemical sensing of nitrite using a copper–titanium oxide composite derived from a hexanuclear complex†
Abstract
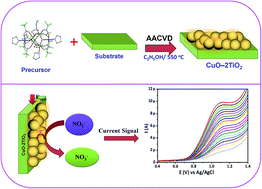
A hexanuclear copper–titanium complex [Cu2Ti4(O)2(OH)4(TFA)8(THF)6]·THF (1) (where TFA = trifluoroacetato, THF = tetrahydrofuran) has been identified for the treatment of copper(II) acetate with titanium(IV) isopropoxide and trifluoroacetatic acid in THF. The physicochemical properties of complex (1) have been inspected by melting point analysis, microanalysis, attenuated total reflectance Fourier transform infrared spectroscopy, thermogravimetry and single crystal X-ray analysis. The “single source” potential of complex (1) has been explored by a solution based aerosol assisted chemical vapor deposition method to fabricate CuO–2TiO2 composite oxide thin films on a fluorinated tin oxide (FTO) conducting glass substrate at 550 °C in ambient air. Thin film characterization such as X-ray powder diffraction, Raman spectroscopy and X-ray photoelectron spectroscopy, energy dispersive X-ray and scanning electron microscopic analyses confirm the evolution of crystalline CuO: a 2TiO2 composite with spherical morphology, with clear grain boundaries and in high purity. Further, the well-characterized film electrode was investigated for electrochemical detection of nitrite ions (NO2−). The fabricated CuO–2TiO2 electrode showed a peak at +1.0 V due to the oxidation of NO2− ions. The limit of detection (LoD) was found to be 0.0166 μM, with the linear range of 10 to 200 μM. Moreover, this present sensor is more selective towards NO2− ions and it did not show any response to other interfering species. This CuO–2TiO2 electrode is a potential candidate for the selective and sensitive detection of toxic NO2− ions towards monitoring the NO2− ion levels in natural water sources for environmental remediation applications.

 Please wait while we load your content...
Please wait while we load your content...