Exploring the thermodynamics and conformational aspects of nicotinic acid binding with bovine serum albumin: a detailed calorimetric, spectroscopic and molecular docking study†
Abstract
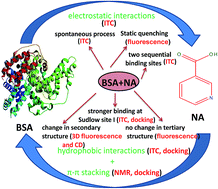
The present study reports comprehensive energetic and conformational aspects of the binding of an antihyperlipidemic drug, nicotinic acid (NA), with a model transport protein, bovine serum albumin (BSA) by calorimetry, light scattering, spectroscopic (absorption, fluorescence, 1H-NMR, and circular dichroism) and molecular docking methods. The calorimetric result reveals that NA binds to BSA in a sequential way with a stronger affinity (∼104 M−1) for the first binding site. The study in the presence of various co-solutes (salt, tetrabutylammonium bromide, sucrose, and surfactants) indicates the significant contribution of electrostatic as well as hydrophobic interactions but insignificant contribution of hydrogen bonding to the binding process. In addition, NA was also observed to bind with BSA through π–π interactions as revealed by 1H-NMR and the molecular docking study. The spectroscopic analysis reveals the formation of a complex via a static quenching mechanism. The presence of two sequential binding events has been successfully explained by calorimetry which has also been supported by the fluorescence study. The changes in the size as well as in the secondary structure of BSA were observed upon binding with NA. The stronger binding of NA at Sudlow site I (subdomain IIA) of BSA has been explored by the molecular docking study in combination with specific site probe experiments. Casting light on such drug–protein interactions helps in better understanding the biomolecular recognition and opens up new approaches in rational drug-design processes.
- This article is part of the themed collection: Editors Collection for RSC Advances - India

 Please wait while we load your content...
Please wait while we load your content...