Hydrogen-bonded bent-core blue phase liquid crystal complexes containing various molar ratios of proton acceptors and donors†
Abstract
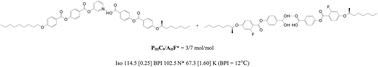
Various hydrogen-bonded (H-bonded) bent-core liquid crystal complexes consisting of pyridyl and benzoic acid derivatives were synthesized and compared with their covalent analogues in this study. The molar ratios of pyridyl and benzoic acid derivatives could be tuned to form various H-bonded liquid crystal (LC) diads (with 1 : 1 molar ratio of H-acceptors T and H-donors D) and complexes (with different molar ratios of T and D). By insertion of H-bonds into different positions of bent-core supramolecules, the mesophasic properties of H-bonded bent-core LC complexes were optimized, which could facilitate the most suitable LC components and compositions to be utilized in H-bonded blue phase (BP) LC complexes. In BPLC complexes, the molar ratios, alkyl chain lengths, the lateral fluoro-substitution and the chiral center of H-bonded bent-core supramolecules would affect the temperature ranges of BPs. Accordingly, H-bonded bent-core complex PIIIC9/AIIF* (3/7 mol mol−1) displayed the widest BPI range of ΔTBPI = 12.0 °C at the correspondent H-acceptor T = PIIIC9 and H-donor D = AIIF*. Since the covalent-bonded bent-core mixture only had narrow ranges of BPIII, we could summarize that the introduction of H-bonds into the bent-core center effectively stabilizes the BPs and easier induces the BPI.

 Please wait while we load your content...
Please wait while we load your content...