Effect of the morphology and phases of WO3 nanocrystals on their photocatalytic efficiency
Abstract
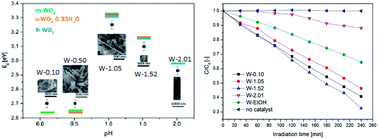
Understanding of the effect of the morphology and crystal phase on photocatalytic efficiency and their precise control are still a great challenge in photocatalysis. In this paper, we use WO3 as an example to study how to control the morphology through understanding the effect of solution pH, EtOH and polymeric surface modulator on the morphology, crystalline phase, band gap and the photocatalytic efficiency of WO3 nanostructures. The photocatalytic efficiency of nano WO3 is a compromise of the band gap, crystal phase, morphology, and the oxidation state. Cuboid-shaped m-WO3 with Eg = 2.7 eV and nanoneedle h-WO3 with Eg = 3.1 eV give high photocatalytic efficiency, while nanowire h-WO3 with Eg = 2.9 eV gives the lowest photocatalytic efficiency. Detailed control of the morphology and crystal phase of nanoscale WO3 were also presented in this paper. pH 1.05 was found to be a transition point for the crystalline phase, crystal size and band gap. pH values lower than 1.05 preferred monoclinic, whereas pH values higher than 1.05 favoured hexagonal WO3 formation. At pH 1.05, the crystal shape changed from cuboid to a fine nanoneedle shape, which was followed by a sudden size decline and an exponential increase in the aspect ratio. At the transition pH, the band gap reached a peak.

 Please wait while we load your content...
Please wait while we load your content...