Evidence of a nanosized copper anodic reaction in an anaerobic sulfide aqueous solution
Abstract
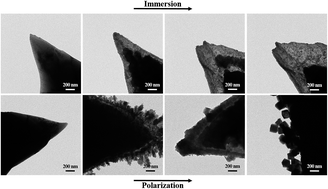
The present paper reports the use of TEM to investigate the electrochemical behavior of a copper subject to the both free corrosion and polarization in a 0.1 M NaCl + 5 × 10−4 M Na2S aqueous solution at the nano scale. The pure copper is found to be transformed into nano-crystalline Cu2S in the thin region of the copper needle in the solution at open circuit conditions. However, a rough Cu2S layer is formed in the active region of electrochemical polarization, which is then converted to the passive CuS layer with a uniform thickness at higher potentials. Upon the continuous increase of an applied potential, cubic CuS particles with sizes of ∼100 nm are precipitated on the needle surface due to the breakdown of the passive layer. Meanwhile, the growth of a large amount of nanosized CuCl particles is also found, indicating that Cl− ions participate in the electrochemical reaction in the transpassive region. It is worth noting that the present work also provides a simple and cost-effective way for the synthesis of copper sulfides (Cu2S and CuS) through electrochemical processes.

 Please wait while we load your content...
Please wait while we load your content...