Phototransformation of tetrazoline oxime ethers: photoisomerization vs. photodegradation†
Abstract
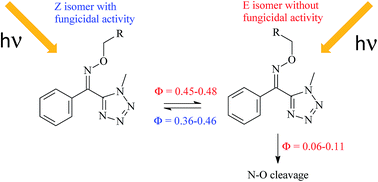
Fungicides showing potent biological activity in green house may have poor activity in the field due to fast photodegradation. This is the case of tetrazoline oxime ethers 1 and 2, active in the Z form, on which very little is known. A comprehensive study was therefore conducted to understand the photochemical behaviour of these compounds. It could be firmly demonstrated that both photoisomerization Z ⇄ E and photodegradation occur, and that interestingly photodegradation only takes place from E forms. Quantum yields of photoisomerization lay within the range 0.38–0.48 and those of E photodegradation in the range 0.06–0.11. During the reaction, the non-fungicidal E forms become the major isomers due to their lower solar light absorptivity than Z forms. The analytical study showed that photodegradation involves the cleavage of the N–O bond and allowed the identification of photoproducts arising from the rearrangement of the iminyl and alkoxyl radicals. Moreover, the proposed mechanism of alkoxyl radical oxidation into corresponding aldehyde and acid was elucidated using a computational study. This fundamental investigation fully explains the fast loss of the fungicidal activity of tetrazoline oxime ethers 1 and 2 in the field.

 Please wait while we load your content...
Please wait while we load your content...