Nitrogen mediated electronic structure of the Ti(0001) surface
Abstract
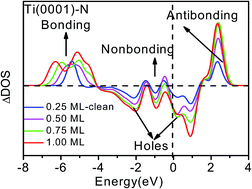
The unusual ability of nitrogen in functionalizing transition metals has tremendous implications for the nitride compounds for chemical, electronic, optical, mechanical, and tribological applications yet a consistent insight into the underlying mechanism remains yet a challenge. A combination of density function theory and photoelectron spectroscopy revealed that the nitrogen atom prefers tetrahedron bonding geometry in the Ti(0001) surface, which derives four additional valence density-of-states:bonding electron pairs, nonbonding lone pairs, electronic holes, and antibonding dipoles. Dipole formation modulates the work function, electron–hole generation opens the bandgap and nonbonding interaction ensures the superlubricity of the N–Ti(0001) skin.

 Please wait while we load your content...
Please wait while we load your content...