A sensitive hydrazine hydrate sensor based on a mercaptomethyl-terminated trinuclear Ni(ii) complex modified gold electrode†
Abstract
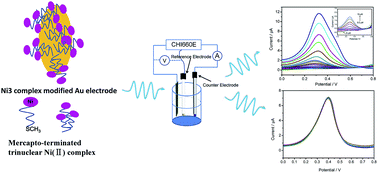
In this study, a novel mercapto-terminated trinuclear Ni(II) complex (Ni3) was synthesized and used as an electrocatalyst for the detection of hydrazine hydrate in real water samples. The as-prepared Ni3 molecule possesses six thiomethyl groups at its periphery and these SCH3 groups can react with Au electrodes to immobilize the Ni3 molecules on their surface through the formation of a self-assembled monolayer. The Ni3-modified Au electrode (Ni3/Au) demonstrates excellent electrocatalytic activity for the oxidation of hydrazine hydrate through a significant decrease in overpotential. The chronoamperometry study shows a diffusion coefficient (D) of 5.82 × 10−5 cm2 s−1 and a catalytic rate constant of 8.57 × 103 M−1 s−1. Using the square wave voltammetry (SWV) technique, this Ni3/Au electrode based hydrazine hydrate sensor exhibits a high sensitivity in quantitative analysis, and its detection limit could be as low as ∼0.07 μM with linearity ranging from 0.2 to 50 μM. In addition, due its good reproducibility, anti-interference performance, and long-term stability, the proposed sensor is capable of detecting trace levels of hydrazine hydrate in real water samples.

 Please wait while we load your content...
Please wait while we load your content...