Mechanism of the antioxidation effect of α-Fe2O3 on silicone rubbers at high temperature†
Abstract
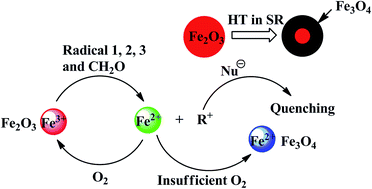
The high temperature resistance of silicone rubber (SR) could be significantly improved by adding hematite (α-Fe2O3). In this study, the variation of α-Fe2O3 and polysiloxanes was investigated by ultraviolet-visible absorption spectroscopy, X-ray diffraction, Mössbauer spectroscopy, and 29Si NMR spectroscopy after SR aging for 12 h in air at 350 °C. The results indicate that Fe3+ is reduced to Fe2+ by organic free radicals (R˙) in the process of aging. Fe3+ captures R˙ to protect the polysiloxanes from being destroyed and is transformed into Fe3O4. Moreover, magnetic Fe3O4 is only found in the inner layer of SR; however, it is not detected in the outer layer. Therefore, a new mechanism of antioxidation is proposed: on the outer part of SR, the reduction–oxidation cycle of Fe3+ is formed because of sufficient oxygen; however, inside the SR, α-Fe2O3 is gradually reduced to Fe3O4.

 Please wait while we load your content...
Please wait while we load your content...