Raman spectroscopic study on the equilibrium of carbon dioxide in aqueous monoethanolamine†
Abstract
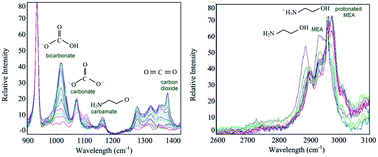
Aqueous phase characterization and thermodynamic modeling of the vapor liquid equilibrium of CO2 in a reactive solvent are important for designing and operating CO2 removal systems. A quantitative method using Raman spectroscopy was applied to determine the absorption capacity and molality of various ionic and molecular species in liquid phase CO2 loaded monoethanolamine (MEA) solutions. Species distribution profiles during absorption were reported for a wide range of CO2 loading. CO2 solubility in aqueous MEA with concentrations varied from 10 to 30 mass% were studied using in situ Raman spectroscopic analysis for pressure ranges from 1 to 50 bar at 303.15, 313.15 and 323.15 K. Vapor liquid equilibrium data for the CO2–MEA–water ternary system was analyzed using the Deshmukh–Mather model.

 Please wait while we load your content...
Please wait while we load your content...