In vivo SAR and STR analyses of alkaloids from Picrasma quassioides identify 1-hydroxymethyl-8-hydroxy-β-carboline as a novel natural angiogenesis inhibitor†
Abstract
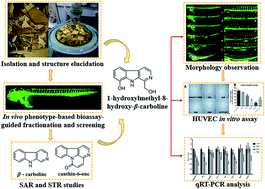
Angiogenesis plays an important role in the development of inflammatory diseases, including cancer, psoriasis and rheumatoid arthritis. In this paper, we conducted a zebrafish bioassay-guided fractionation of Picrasma quassioides and identified twenty alkaloids from the anti-angiogenic fraction, including four new ones (1–4). In addition, in vivo relationship analyses of the structure and anti-angiogenic activity/toxicity led to the conclusion that the skeleton of alkaloids as well as the positions and properties of the substituents are pivotal to their activity and toxicity. Furancanthin (1) is the first-reported furan-fused canthin-6-one with an unprecedented highly-conjugated pentacyclic skeleton. 1-Hydroxymethyl-8-hydroxy-β-carboline (3) was found to have the most potent anti-angiogenic activity and the lowest toxicity in vivo, whose anti-angiogenic activity was also confirmed in vitro. Further qRT-PCR analysis revealed that the kdr, kdrl signaling axle in the VEGF–VEGFR pathway and the angpt2b, tek in the ANGPT–TEK pathway seemed to be involved in the anti-angiogenic activity of compound 3.

 Please wait while we load your content...
Please wait while we load your content...