Characterization of regio- and stereo-selective sulfation of bufadienolides: exploring the mechanism and providing insight into the structure–sulfation relationship by experimentation and molecular docking analysis†
Abstract
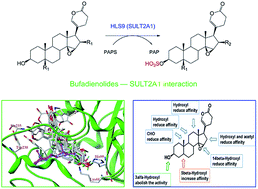
Bufadienolides are a major class of bioactive compounds derived from amphibian skin secretion. Recent studies demonstrate that bufadienolides have a promising role in targeted cancer chemotherapy. However, extensive metabolism and inactivation strongly restrict the clinical applications of bufadienolides. This study aimed to systematically characterize the sulfation of six representative bufadienolides (including bufalin, resibufogenin, cinobufagin, bufotalin, telocinobufagin and deacetylcinobufagin) in amphibian skin secretion and to provide insight into the structure–sulfation relationship by experimentation and molecular docking analysis with series of bufadienolides and derivatives. For all the six representative bufadienolides, one corresponding monosulfate was detected in the incubation mixtures. The sulfates were accurately identified as bufadienolides 3-O-sulfates by NMR and HPLC-MSn techniques. Reaction phenotyping studies using human recombinant sulfotransferase (SULT) and liver S9 demonstrated that SULT2A1 mediated the formation of bufadienolide 3-O-sulfate with a high specific selectivity. Further kinetic evaluation demonstrated that deacetylcinobufagin could be used as a preferred probe of SULT2A1. The regio- and stereo-selective sulfation properties of SULT2A1 and the structural variation effects of bufadienolides were investigated by docking analysis, which revealed the significance of appropriate molecule orientation and hydrophobic interactions of motifs with SULT2A1 His99 residues. Additionally, significant differences between humans and animal species were observed in the sulfation of bufalin and resibufogenin. This study provided important data for elucidating the mechanisms of bufadienolide sulfation and leads to a better understanding of the bufadienolide–SULT interaction which can be further used in preclinical development and rational use of bufadienolides.

 Please wait while we load your content...
Please wait while we load your content...