Probing molecular basis of single-walled carbon nanotube degradation and nondegradation by enzymes based on manganese peroxidase and lignin peroxidase†
Abstract
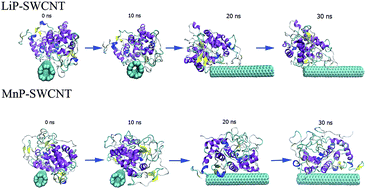
Increasing evidence has shown that carbon nanotubes (CNTs) present adverse effects on the environment and human health, which stresses the importance of exploring CNT biodegradation. In this study, we describe the molecular basis of single-walled carbon nanotube (SWCNT) biodegradation using a CNT-degrading enzyme (manganese peroxidase, MnP) and a CNT-nondegrading enzyme (lignin peroxidase, LiP, from Phanerochaete chrysosporium) with similar catalytic cycles. Our results showed that SWCNT impeded the native conformational changes in free LiP by anchoring its loop regions to avoid the degradation. In contrast, SWCNT did not limit conformational transitions in MnP and might induce larger conformational fluctuations than in free MnP by interacting with its helical and loop regions, providing the molecular basis of SWCNT degradation. SWCNT slightly affected the secondary structures and the mean smallest distances between residue pairs in LiP and MnP. These findings provide a better understanding of the biodegradation mechanism of CNTs and pre-estimating the biodegradation potential of CNTs and are useful when developing more promising CNT-degrading enzymes.

 Please wait while we load your content...
Please wait while we load your content...