Lipase-catalyzed synthesis of oxidation-responsive poly(ethylene glycol)-b-poly(β-thioether ester) amphiphilic block copolymers†
Abstract
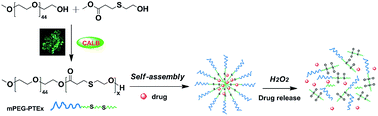
A series of novel oxidation-responsive amphiphilic diblock copolymers with β-thioether ester groups were synthesized via the one-step lipase-catalyzed polycondensation of methyl 3-((2-hydroxyethyl)thio)propanoate (MHETP) with monomethoxy poly(ethylene glycol) (mPEG) using immobilized lipase B from Candida antarctica (CALB) as the catalyst. The amphiphilic poly(ethylene glycol)-b-poly(β-thioether ester) (mPEG-b-PTE) diblock copolymers could self-assemble to form nanosized micelles in aqueous solution with low critical micelle concentration (CMC). The micelles were able to undergo a H2O2-triggered disassembly due to the oxidizable thioether groups and degradable ester groups in the hydrophobic PTE core. The oxidation-responsive behaviour of the micelles was further investigated by dynamic light scattering (DLS) and transmission electron microscopy (TEM). Nile Red, a hydrophobic model drug, was encapsulated into the polymeric micelles and showed fast release upon the addition of H2O2. Cell cytotoxicity tests indicated that the micelles had low cytotoxicity toward HeLa cells. The oxidation-insensitive poly(ethylene glycol)-b-poly(ε-caprolactone) (mPEG-b-PCL) diblock copolymer was also prepared for comparison. All these findings demonstrated the potential of mPEG-b-PTE diblock copolymers as a promising oxidation-responsive nanocarrier for controlled drug delivery.

 Please wait while we load your content...
Please wait while we load your content...