Bifunctional thiourea catalyzed asymmetric Michael addition of anthrone to methyleneindolinones†
Abstract
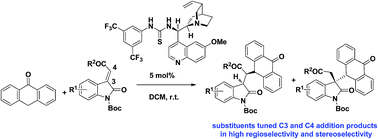
Biologically significant hybrid derivatives of oxindoles and anthrones were prepared in high yield, high regioselectivity and high stereoselectivity via bifunctional thiourea catalyzed asymmetric conjugate addition of anthrone to methyleneindolinones. The regioselectivity and stereoselectivity could be finely tuned via change of substituents in methyleneindolinones.

 Please wait while we load your content...
Please wait while we load your content...