Donor–acceptor copolymers containing phthalazinone–thiophene structure synthesized by low-cost copper-catalyzed Ullmann polymerization†
Abstract
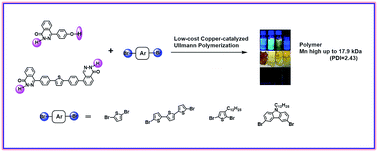
Herein, we present low-cost copper-catalyzed Ullmann polycondensation of phthalazinone based monomers containing both OH and NH groups, which provides access to polyarylethers and conjugated polymers. The synthesis of the polymers was accomplished by a combination of low activity thiophene and carbazole monomers. In particular, it was shown that a stronger base (Cs2CO3 > K2CO3) can efficiently polymerize the low activity thiophene monomer with the phthalazinone based monomer, to obtain high number-average molecular weight, up to 17.9 kDa (PDI = 2.43). Moreover, in this study, the result of reaction process monitoring revealed that the Ullmann polymerization was in favour of the radical mechanism. The reaction activity of di-halogenated monomers was found to be a crucial factor for achieving a high molecular weight polymer. Thus, the number-average molecular weight of donor–acceptor copolymers containing the phthalazinone–thiophene structure ranged from 2.2 to 9.6 kDa. The new copolymer structures were confirmed by 1H-NMR, FT-IR and GPC. Their thermal, optical, electrochemical and X-ray diffraction (XRD) properties, and density functional theory (DFT) calculations, were investigated in detail. The results show that the thiophene unit is beneficial to the π–π interaction between the polymer chains, compared to carbazole and phthalazinone units.

 Please wait while we load your content...
Please wait while we load your content...