Kinetic, isotherm and thermodynamic studies of adsorption behaviour of CNT/CuO nanocomposite for the removal of As(iii) and As(v) from water†
Abstract
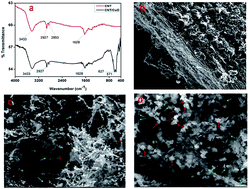
A CNT/CuO nanocomposite prepared by precipitation method was characterized through FT-IR, XRD, SEM, TGA, BET and Raman spectroscopy and utilized as a nanoadsorbent for the adsorption of As(III)/As(V) from water where maximum uptake capacities of 2267 μg g−1 for As(III) and 2395 μg g−1 for As(V) were achieved. This CNT/CuO nanocomposite is a better alternative to known conventional adsorbents because it has a high surface area (480 m2 g−1) and maximum uptake capacities were achieved at ambient temperature (30 °C) and near neutral pH (pH 7 for As(III) and 5 for As(V)). Kinetic studies indicated that a pseudo-second-order kinetic model described the kinetic data. The mass transfer and intraparticle diffusion studies suggested that both external mass transfer and intraparticle diffusion steps contributed to the rate controlling step. The Boyd model suggested that intraparticle diffusion was the main rate controlling step. Isotherm studies were conducted and it was found that the equilibrium data followed the Langmuir isotherm which indicated that the adsorption was a monolayer and all the binding sites were energetically equivalent. The D–R isotherm revealed that the adsorption was chemisorption. The thermodynamic studies revealed that the adsorption was spontaneous because ΔG0 was negative and the reaction was endothermic due to the positive value of ΔH0. XPS analysis revealed that CNT/CuO not only adsorbed As(III)/As(V) but also oxidized highly toxic As(III) to less toxic As(V) which is an added advantage of CNT/CuO as an adsorbent.

 Please wait while we load your content...
Please wait while we load your content...