Synthesis of colloidal MnO2 with a sheet-like structure by one-pot plasma discharge in permanganate aqueous solution†
Abstract
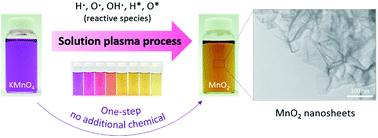
Stable colloidal MnO2—consisting of MnO2 with a sheet-like structure—was synthesized by solution plasma process (SPP). The synthesis was completed in one-step by discharging the plasma in potassium permanganate (KMnO4) aqueous solution without using any dispersants or stabilizers. An alteration of the manganese oxide oxidation state, from MnO4− to MnO2 as a function of the discharge time, was investigated by UV-vis spectroscopy. Morphology and elemental constituents were observed by TEM and EDS mapping. Results indicated that the discharge time was an important feature for the formation of MnO2 in the SPP system. Specifically at neutral pH (pH = 7), MnO4− was completely reduced to MnO2 within a discharge time of 18 min. After that the obtained MnO2 was converted rapidly to Mn2+. To better understand the possible pathways of MnO2 formation by the SPP, we compared aspects of the reaction under different pH conditions. Formation of MnO2 under additional, controlled pH conditions, i.e. 2 and 12, was studied. Results suggested that hydrogen species played a key role for the reduction of MnO4− in the water-based SPP system. In comparison to the existing routes for the synthesis of MnO2 nanosheets with a single or low number of layers, the SPP holds excellent promise as an effective alternative means regarding its simplicity, time-energy preserving, and scalability.

 Please wait while we load your content...
Please wait while we load your content...