Ester production from bio-based dicarboxylates via direct downstream catalysis: succinate and 2,5-furandicarboxylate dimethyl esters
Abstract
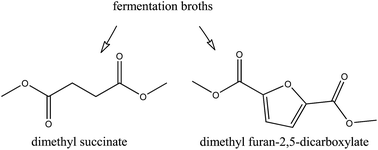
Two culture broths, one containing succinate produced de novo by Corynebacter glutamicum and the other containing 2,5-furandicarboxylate produced by whole cell biotransformation of 5-(hydroxymethyl)furfural (HMF) by a recombinant Pseudomonas putida, were used for dimethyl ester production. For anion exchange, they were characterized for competing organic anions (i.e., other carboxylates) and inorganic anions (phosphate, sulfate and chloride), which affect capturing of the target dicarboxylate via sorption. For the analysis of the sorption process, independent multicomponent column experiments using mimicked mixtures of the respective target building blocks with organic anions, inorganic anions and actual fermentation broth were performed. In the case of succinate, breakthrough profiles and column capacities showed that α-ketoglutarate, malate and other fermentation impurities reduced sorption capacity. For 2,5-furandicarboxylate, the effect of impurities in sorption was less pronounced, with residual HMF eluting without any apparent ionic interaction. After sorption, upgrading via alkylation from mimicked and bio-based broth was successfully carried out, producing the respective succinate and 2,5-furandicarboxylate dimethyl esters. The yield towards dimethyl succinate was reduced from 0.98 to 0.66 mol ester per mol carboxylate due to the presence of fermentation impurities, which were also esterified in good yields. The final yield of dimethyl 2,5-furandicarboxylate ranged between 0.75 and 0.77 mol ester per mol carboxylate for both pure and raw bio-based sorbed furandicarboxylate. Esterification kinetics correlate well with the acidity of the carboxylates and impurities.

 Please wait while we load your content...
Please wait while we load your content...