A mechanistic study of carbonyl activation under solvent-free conditions: evidence drawn from the synthesis of imidazoles†
Abstract
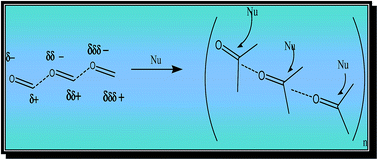
Syntheses of various imidazoles and their derivatives, imidazole N-oxides and 1-hydroxyimidazole 3-oxides, from sterically different dicarbonyl moieties provided insights into the self-catalytic effect of the condensed phase reactions of carbonyl compounds. The self-catalytic activity in solvent-free multi-component syntheses was investigated using a combination of methods viz., reactivity, spectroscopy and theory. While IR spectroscopic studies revealed that reacting molecules were polarised in bulk, quantum mechanical calculations of associated HCHO monomers suggest an increase in the average dipole moment of each monomer and provide evidence for the presence of cooperative effects. A comparative study of the kinetics of un-catalysed and catalysed reactions with the help of HPLC provided insights into the mechanism.

 Please wait while we load your content...
Please wait while we load your content...