Synthesis, preclinical evaluation and molecular modelling of macrocyclic appended 1-(2-methoxyphenyl)piperazine for 5-HT1A neuroreceptor imaging†
Abstract
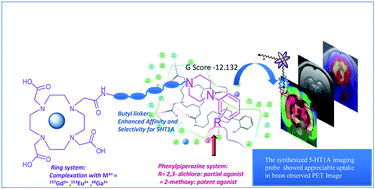
5-HT1A receptors are known to be implicit in a number of neuropsychiatric fluctuations related to mood and anxiety. Their visualization in the human brain using PET, SPECT or MRI is of great importance in the management and treatment of neurological disorders. The present work focuses on the metal complexes (Gd3+, Eu3+ and Ga3+) of DO3A-butyl-MPP to be used as brain (cerebral cortex, hippocampus, and amygdala) imaging agents using different modalities. Synthesis of 2,2′,2′′-(10-(2-(4-(4-(2-methoxyphenyl)piperazin-1-yl)butylamino)-2-oxoethyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl)triacetic acid (DO3A-butyl-MPP) was achieved by conjugating the chloroacetylated derivative of 1-(2-methoxyphenyl)piperazine-butylamine with trisubstituted cyclen with subsequent cleavage with trifluoroacetic acid (TFA). The resulting compound was then labeled with GdCl3 and 68GaCl3 to perform MRI (relaxivity studies) and PET respectively. The longitudinal relaxivity (r1), and transverse relaxivity (r2), were determined to be 6.66 and 11.486 mM−1 s−1 respectively on 7 T at 21 °C. The SD (Sprague Dawley) rat brain uptake was 3.91% ID per g (percentage of the injected dose per gram) at 30 min post injection. Homology modeling and docking studies at the shallow antagonist binding pocket of 5-HT1A show a high G score of −12.132 that confers high binding of the ligand at the target receptor.

 Please wait while we load your content...
Please wait while we load your content...