Electrochemical study of ivabradine hydrochloride ion selective electrodes using different ionophores
Abstract
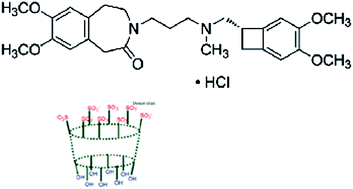
Three novel ivabradine hydrochloride (IH) selective electrodes were investigated with dioctylphthalate (DOP) as a plasticizer in a polymeric matrix of polyvinyl chloride (PVC). Sensor 1 was fabricated using tetrakis(4-chlorophenyl)borate (TpClPB) as an anionic exchanger without incorporation of an ionophore. Sensors 2 and 3 were constructed using 4-tertary butyl sulfocalix-8-arene and 2-hydroxypropyl β-cyclodextrin as an ionophore. Linear responses of IH within the concentration ranges of 10−5 to 10−2 mol L−1 were obtained using sensors 1, 2 and 3, respectively. Near Nernstian slopes of 56.39, 56.1.50 and 57.7 mV/decade over the pH range of 4–8 were observed. The selectivity coefficients of the developed sensors indicated excellent selectivity for IH. The proposed sensors displayed useful analytical characteristics for the determination of IH in bulk powder, pharmaceutical formulation and biological fluids (plasma, homogenized rat heart tissue).

 Please wait while we load your content...
Please wait while we load your content...