Following heterogeneous nucleation of CO2 on H2O ice nanoparticles with microsecond resolution
Abstract
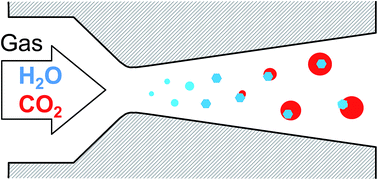
Heterogeneous nucleation of CO2 onto H2O ice particles may play an important role in proposed innovative CO2 capture technologies, as well as in the formation of Martian clouds. In this work we follow the nucleation/condensation of CO2/H2O gas mixtures with microsecond resolution in supersonic Laval nozzles using pressure trace measurement (PTM) and small angle X-ray scattering (SAXS). The latent heat release detected by the PTM reveals that the first phase transition in the expanding CO2/H2O mixture is the formation of H2O ice particles by the homogeneous nucleation/condensation and freezing of H2O. This is followed by the heterogeneous nucleation and growth of CO2 on the H2O ice particles. The onset conditions for heterogeneous nucleation, i.e. the partial pressure of CO2 and temperature from PTM and the radius of gyration of the H2O ice particles from SAXS, were determined in the temperature range 124 to 146 K and for particles with radii of gyration in the range of 2.1 to 4.3 nm. The onset conditions suggest that the heterogeneous nucleation of CO2 may start from the supercooled liquid phase under our conditions. Downstream of the onset point, the partial pressure of CO2 and temperature rapidly approach the vapor–solid equilibrium line of CO2, demonstrating that even if CO2 condensation is initiated by heterogeneous nucleation of the liquid phase, it proceeds via growth of the solid.

 Please wait while we load your content...
Please wait while we load your content...