Facile synthesis of 5-hydroxymethylfurfural: a sustainable raw material for the synthesis of key intermediates toward 21,23-dioxaporphyrins†
Abstract
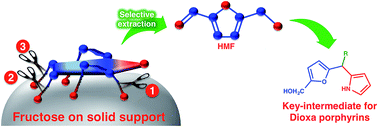
In a simple and conceptually designed method for the dehydration of fructose on a solid support, 5-hydroxymethylfurfural (HMF) was synthesized in more than 95% isolated yield from fructose under very mild conditions at room temperature. The loading of fructose on the solid support reduced the intermolecular interactions between fructose/intermediates, diminished the formation of polymeric material (humin) and increased the selectivity towards HMF formation. The synthesized HMF was readily converted into key intermediates toward the simpler synthesis of 21,23-dioxaporphyrins.

 Please wait while we load your content...
Please wait while we load your content...