Dispersive suspended-solidified floating organic droplet microextraction of nonsteroidal anti-inflammatory drugs: comparison of suspended droplet-based and dispersive-based liquid-phase microextraction methods
Abstract
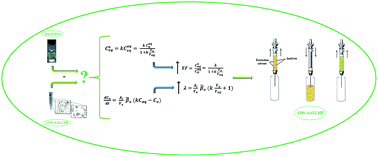
Herein, a dispersive suspended-solidified floating organic droplet microextraction method was first developed to improve some limitations of droplet-based microextraction methods including long extraction times and uncertainties in the collection of low volume of extraction solvents coupled with high-performance liquid chromatography (HPLC). To the best of our knowledge, neither the extraction efficiency of droplet- and dispersive-based liquid-phase microextraction methods, under disperser solvent-free conditions, nor their ability to pre-concentrate nonsteroidal anti-inflammatory drugs (NSAIDs) from bio-fluid samples has been investigated so far. In this way, two droplet-based (directly suspended droplet and dispersive suspended), two solidified droplet-based (directly suspended-solidified floating organic droplet and dispersive suspended-solidified floating organic droplet), and two dispersive-based (air-assisted liquid–liquid and ultrasound-assisted emulsification) microextraction methods were studied and compared for the determination of three NSAIDs as model analytes. The influential parameters on the extraction efficiency of all methods were critically investigated and compared thermodynamically and kinetically. However, considering some advantages such as higher enrichment factors, shorter extraction time and simplicity in operation, the best results were obtained using the low density solvent-based air-assisted liquid–liquid microextraction (LDS-AALLME) method, which employed 65.0 μL of n-octanol as extraction solvent, 5 mL of sample at pH 2.5, without salt addition, and 10.0 extraction cycles (during 40 s). This method was validated with satisfactory results including low limits of detection (1.1 to 1.7 μg L−1), wide linear dynamic ranges (3.5 to 2448 μg L−1), acceptable recoveries (94 to 102%) and relative standard deviations (in terms of repeatability, <7.9%). Finally, the LDS-AALLME method coupled to HPLC was successfully applied for determination of ibuprofen, mefenamic acid and sodium diclofenac in human plasma and urine samples.

 Please wait while we load your content...
Please wait while we load your content...