Enhancement of electron-donating character in alkylated monopyrrolo-tetrathiafulvalene derivatives†
Abstract
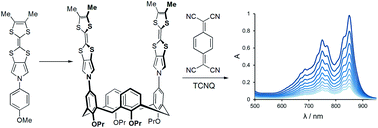
Herein we report the first synthesis of a monopyrrolo-tetrathiafulvalene (MPTTF) with two alkyl substituents, performed using a triphenylphosphine-mediated coupling reaction. An Ullmann-type N-arylation reaction was used to prepare a calixarene-based bis-MPTTF receptor, as well as an arylated mono-MPTTF derivative. Complexation studies with electron-deficient compounds showed strongly enhanced affinity of the alkyl-substituted MPTTF derivatives in comparison with the non-substituted MPTTF analogues.

 Please wait while we load your content...
Please wait while we load your content...