Detection of copper(ii) and aluminium(iii) by a new bis-benzimidazole Schiff base in aqueous media via distinct routes†
Abstract
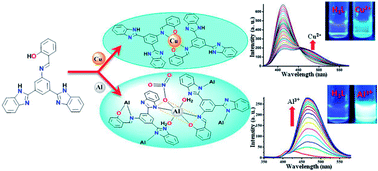
The synthesis is described of a new bis-benzimidazole appended Schiff base ligand [2-(3,5-bis(1H-benz-imidazol-2-yl)-phenyliminomethyl)phenol] (H3L) followed by its thorough characterization by elemental analyses, spectroscopic studies (FT-IR, 1H, 13C NMR, ESI-MS, UV/vis, fluorescence) and X-ray single crystal analyses. The excellent selectivity of H3L towards Cu2+ and Al3+ via distinct responses in mixed aqueous conditions was established by spectroscopic studies. The selective detection of Cu2+ due to the creation of complex 1 in which Cu2+ occupied only the salen-type N2O2 coordination site and the strong binding with Al3+ through both benzimidazole and salen moieties to yield complex 2 was confirmed by various studies. The disparate detection of these cations by H3L was substantiated by comparative studies, performed under analogous conditions, of the precursor compound 3,5-bis(1H-benzimidazol-2-yl)-aniline (BBA). The distinct interaction of H3L with Cu2+ and Al3+ and the sensing mechanisms were investigated in detail by spectroscopic studies and were supported by theoretical studies (Density Functional Theory). It has been clearly shown that the use of appropriate substituents may facilitate the design and development of probes suitable for the real time detection of more than one analyte.

 Please wait while we load your content...
Please wait while we load your content...