Acrylamide modified poly(vinyl alcohol): crystalline and enhanced water solubility
Abstract
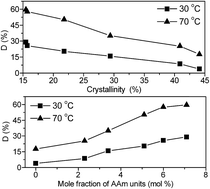
Acrylamide (AAm) modified polyvinyl alcohol (PVA) with enhanced water solubility and tunable tacticity and crystallinity was prepared by alcoholysis of vinyl acetate (VAc) and acrylamide copolymers. The chemical structures and performance of VAc–AAm copolymers and AAm-modified PVA were measured by FTIR, UV-vis, XRD, elemental analysis as well as rheometry. FTIR and XRD analysis reveals that AAm units in PVA chain can reduce the stereoregularity effectively, and suppress the crystallinity. And a decreasing linear relationship between crystallinity and AAm mole fractions is observed. Furthermore, the influence of AAm modification on the water solubility of PVA was studied, and a significant enhancement either at low temperature (30 °C) or at high temperature (70 °C) was achieved through AAm modification. Moreover, the rheological investigation suggested that the relative strength of the hydrogen bonding interactions existing between PVA chains was weakened, while those between PVA chains and water molecules, to some extent, were enhanced after AAm modification. The method presented is an easy but promising way to prepare PVA that can have good water solubility even at low temperature while exhibiting high degrees of alcoholysis.

 Please wait while we load your content...
Please wait while we load your content...