Low-viscosity ether-functionalized pyrazolium ionic liquids based on dicyanamide anions: properties and application as electrolytes for lithium metal batteries†
Abstract
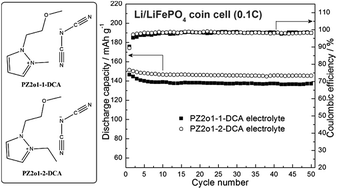
Four new ether-functionalized pyrazolium ionic liquids (ILs) based on dicyanamide (DCA) anions are synthesized and characterized. The physical and electrochemical properties of these ILs, including melting point, thermal stability, viscosity, conductivity and electrochemical stability are investigated. All these ILs are liquids and their viscosities are lower than 40 mPa s at 25 °C. Though the four IL electrolytes with 0.6 mol kg−1 LiDCA have good chemical stability against lithium metals, only 1-(2-methoxyethyl)-2-methylpyrazolium dicyanamide (PZ2o1-1-DCA) and 1-(2-methoxyethyl)-2-ethylpyrazolium dicyanamide (PZ2o1-2-DCA) electrolytes can be used as electrolytes for lithium metal batteries due to the formation of a beneficial SEI film on the lithium metal during the cycling test of a symmetric lithium cell. At room temperature, Li/LiFePO4 coin cells using the two electrolytes show good cycling performance at 0.1C, and the cell using PZ2o1-2-DCA electrolyte shows better rate performance.

 Please wait while we load your content...
Please wait while we load your content...