Effects of an acid–alkaline environment on the reactivity of 5-carboxycytosine with hydroxyl radicals†
Abstract
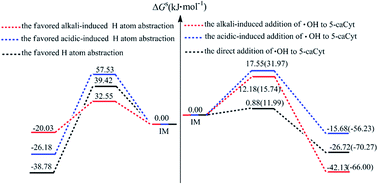
A hydroxyl radical (˙OH) is produced in biological systems by external or endogenous agents. It can damage DNA/RNA by attacking pyrimidine nucleobases through an addition reaction and H-atom abstraction. However, the correlation study for the new cytosine derived DNA modification (5-carboxycytosine, 5-caCyt) remains scarcely existent. Here three distinct groups of mechanisms for 5-caCyt with ˙OH by the CBS-QB3 approach have firstly been explored: the direct reaction (paths R1–R6), acidic (paths R1′–R3′, R5′, R6′), and alkaline (paths R1′′–R5′′)-induced processes. It indicates that the addition of ˙OH to the C5![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C6 double bond of 5-caCyt is more favourable in neutral, acidic and alkaline conditions, and the ΔGs≠ value of the C5 channel is a little higher than that of the C6 route, which agrees with the tendencies observed experimentally. Moreover, the H5 abstraction in alkaline media might be competitive with the addition reactions, having a ΔGs≠ value of 32.55 kJ mol−1, which is only 17–20 kJ mol−1 more energetic than for the addition reactions. In addition, the ΔGs≠ values of the ˙OH reactions are slightly lower for the neutral or deprotonated systems than for the N3-protonated 5-caCyt, implying that the reaction trends are a little enhanced. Our results give a possible new insight on 5-caCyt in the presence of ˙OH for experimental scientists.
C6 double bond of 5-caCyt is more favourable in neutral, acidic and alkaline conditions, and the ΔGs≠ value of the C5 channel is a little higher than that of the C6 route, which agrees with the tendencies observed experimentally. Moreover, the H5 abstraction in alkaline media might be competitive with the addition reactions, having a ΔGs≠ value of 32.55 kJ mol−1, which is only 17–20 kJ mol−1 more energetic than for the addition reactions. In addition, the ΔGs≠ values of the ˙OH reactions are slightly lower for the neutral or deprotonated systems than for the N3-protonated 5-caCyt, implying that the reaction trends are a little enhanced. Our results give a possible new insight on 5-caCyt in the presence of ˙OH for experimental scientists.

 Please wait while we load your content...
Please wait while we load your content...