Electrical and magnetic properties of FeS2 and CuFeS2 nanoplates†
Abstract
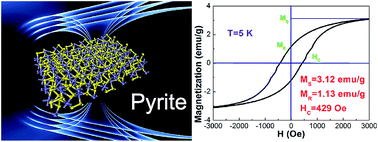
The properties of nanomaterials are always connected with their crystal morphologies of the crystals. Here, we report the synthesis of square-like high crystalline iron pyrite (FeS2) and chalcopyrite (CuFeS2) nanoplates with an average dimension of 70 nm by a developed hydrothermal process. TEM, XRD and Raman spectra characterization indicate that the nanoplates are high crystalline and pure phase. The optical band gaps of the FeS2 and CuFeS2 nanoplates are 0.97 and 0.52 eV, respectively. The electrical measurements indicate that the nanoplates have good electrical conductivity. With the dangling bonds at interface, the as-made nanoplates exhibit abnormal strong ferromagnetic behavior both at room temperature and low temperature (5 K). These nanoplates with unique morphology, high quality and good performance show huge potential in inexpensive nanoelectronics, such as solar cells and magnetic area.


 Please wait while we load your content...
Please wait while we load your content...