Synthesis of 4,5,6-trisubstituted-1,3-dihydroisobenzofurans by virtue of palladium-catalyzed domino carbopalladation of bromoenynes and internal alkynes†
Abstract
An efficient hetero-annulation protocol has been developed for the construction of 4,5,6-trisubstituted-1,3-dihydroisobenzofurans via palladium-catalyzed domino carbopalladation of bromoenynes and internal alkynes. The reaction followed domino intramolecular Heck cyclization (5-exo-dig) and termination of the resulting diene with an internal alkyne to give highly substituted isobenzofurans in moderate to good yields.
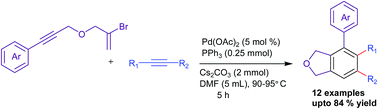

 Please wait while we load your content...
Please wait while we load your content...